COVID-19 Neutralizing Antibody Detection Kit
Intended Use
This product uses lateral flow chromatographic immunoassay for the qualitative detection of neutralizing antibodies in human serum, plasma, or whole blood from people who have received vaccination or who have been recovered from COVIV-19.
SUMMARY
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic virus carriers can also be infectious sources. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are also found in some cases.
PRINCIPLE
The SARS-CoV-2 neutralizing antibodies are the protective antibodies which produced by the human body after vaccination or viral infection. This kit uses the ACE2 receptor to competitively combine to viral S-RBD antigen with neutralizing antibodies. It is suitable for detecting the immune effect after vaccination or viral infection. The test strip consists of: 1) a burgundy-colored conjugate pad containing SARS-COV-2 S-RBD antigen conjugated with colloid gold and mouse IgG-gold conjugates, 2) a nitrocellulose membrane strip containing a test line (T line) and a control line (C line). The T line is pre-coated with ACE2 receptor. The C line is pre-coated with goat anti mouse IgG. When an adequate volume of specimen is dispensed into the sample loading hole on a test card, the specimen migrates by capillary action across the strip. If neutralizing antibodies are present in the specimen, they will bind to the S-RBD antigen on the colloid gold, and block the binding site of ACE2 receptors. Therefore, the strip will have diminished color intensity at T line or even absence of T line. If the specimen doesn’t contain neutralizing antibodies, the S-RBD antigen on the colloid gold will bind to the ACE2 receptors with maximum efficiency. Therefore, the strip will have enhanced color intensity at T line.
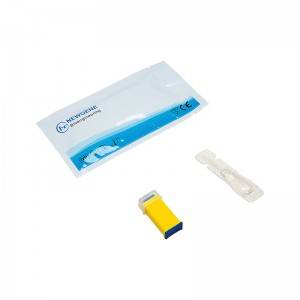
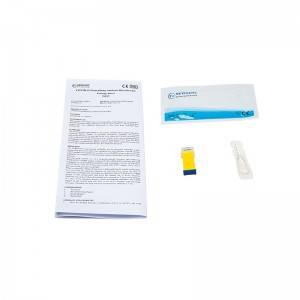
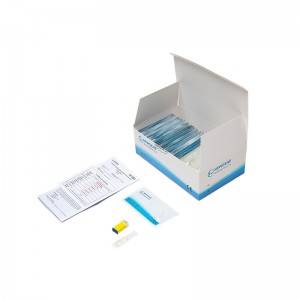
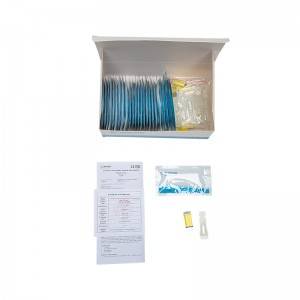
COMPOSITION
1. Test Card
2. Blood Sampling Needle
3. Blood Dropper
4. Buffer Bulb
STORAGE AND STABILITY
1. Store the product package at temperature 2-30°C or 38-86°F, and avoid exposure to sunlight. The kit is stable within the expiration date printed on the label.
2. Once an aluminum foil pouch is opened, the test card inside should be used within one hour. Prolonged exposure to hot and humid environment may cause inaccurate results.
3. The lot number and the expiration date are printed on the label.
WARNINGS AND PRECAUTIONS
1. Read the instructions for use carefully before using this product.
2. This product is for self-test use by non-professional users or professional use.
3. This product is applicable to whole blood, serum, and plasma samples. Using other sample types may cause inaccurate or invalid test results.
4. Please make sure that a proper amount of sample is added for testing. Too much or too little sample amount may cause inaccurate results.
5. If the test line or control line is out of the test window, do not use the test card. The test result is invalid and retest the sample with another one.
6. This product is disposable. DO NOT recycle used components.
7. Dispose of used products, samples, and other consumables as medical wastes under relevant regulations.